HALOGENATION OF ENOLS AND ENOLATES
Another chemical consequence of the enolization of aldehydes and ketones is their halogenation in alpha position.
It can be effected either from the enol or the enolate.
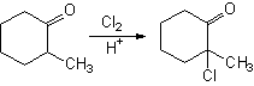
How is 2-methylcyclohexanone halogenated?
It bears two alpha positions to the carbonyl, however...
Why the reaction is regioselective?
Experimentally it is found:
1. The reaction rate is first order in the substrate.
This means that the halogen has nothing to do with the reaction rate. The reaction is actually independent of the used halogen
(Cl2, Br2 or I2).
2. The reaction displays a positive isotopic effect
(kH/kD > 1).
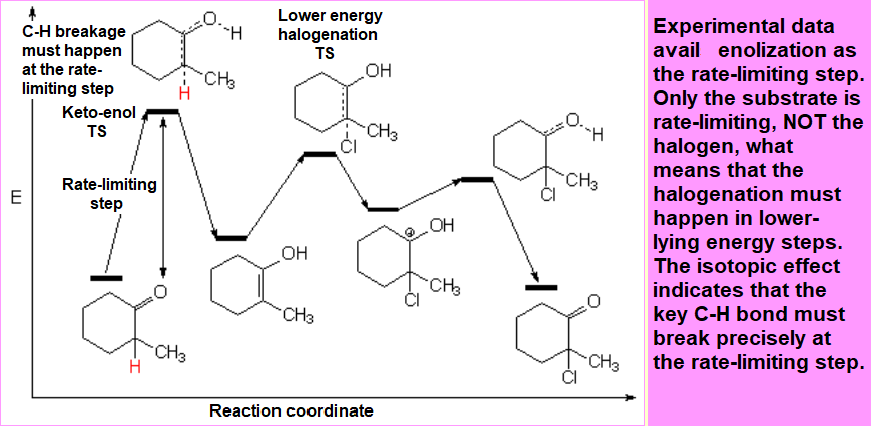
In order to device a reasonable mechanism one has to bear in mind that the only species responsible for the rate-limiting step must be the substrate, NOT the halogen.
Besides, the key C-H cleavage must also happen in the rate-limiting step because otherwise the sotopic effect wouldn't be observed.
Enol formation must thus be the rate-limiting step (highest TS): It only implies the substrate and the Calpha-H bond breakage happens there.
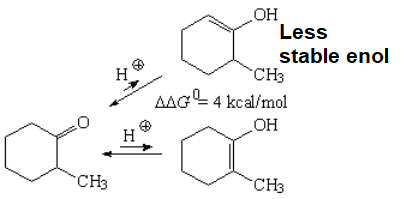
From the two possible enols, the most substituted is the most stable one.
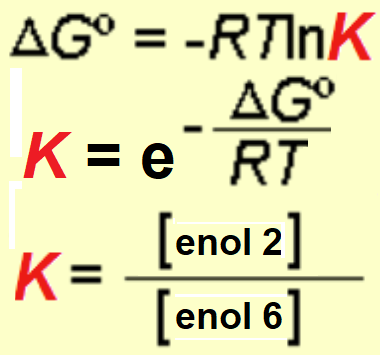
An energy difference of 4 kcal/mol was measured between the two possible enols what means that, at room temperature, the ratio of the most stable enol (enol-2) is higher than 99.5%.
Use the equations to check that out.
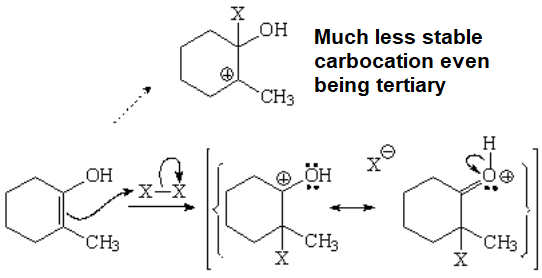 This is another example about the apparently contradictory capabilities of an oxygen to stabilize adjacent positive charges.
This is another example about the apparently contradictory capabilities of an oxygen to stabilize adjacent positive charges.
DON'T FORGET: The oxygen of an alcohol or an ether is a net electron donor when attached to a sp2 carbon like that of a carbocation.
Click here to recall other examples.
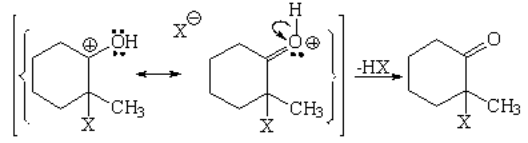
The halogen behaves as an electrophile as it couldn't be otherwise. One halogen "steals" electrons from the double C=C bond while the other departs. The two of them get complete octets.
The attack to the enol might proceed in two ways but it takes the lowest energy path, i.e. through the least unstable carbocation.
In the last step a proton is lost forming HX with the remaining halide and yielding 2-chloro-2-methyl-cyclohexanone.
The whole qualitative energy diagram should be like this one:
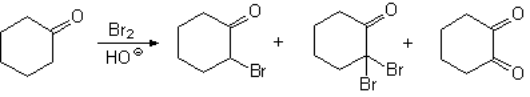
The halogenation of aldehydes or ketones in basic media, through their enolate ions, is very problematic because usually renders complex product mixtures.
Polyhalogenation cannot be avoided!!!
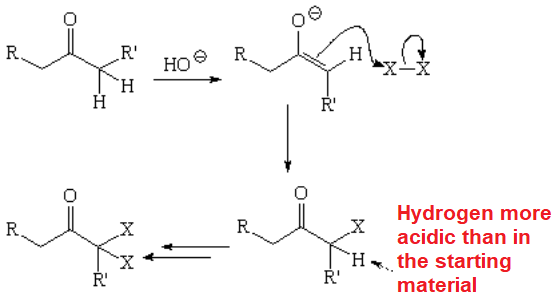
The result is a mixture as in this example:
The alpha-gem-dihaloderivative can even evolve into a diketone following a reaction that you already know.
 This is another example about the apparently contradictory capabilities of an oxygen to stabilize adjacent positive charges.
This is another example about the apparently contradictory capabilities of an oxygen to stabilize adjacent positive charges.