CIS -TRANS (E/Z) ISOMERISM OF ALKENES.
RELATIVE STABILITY
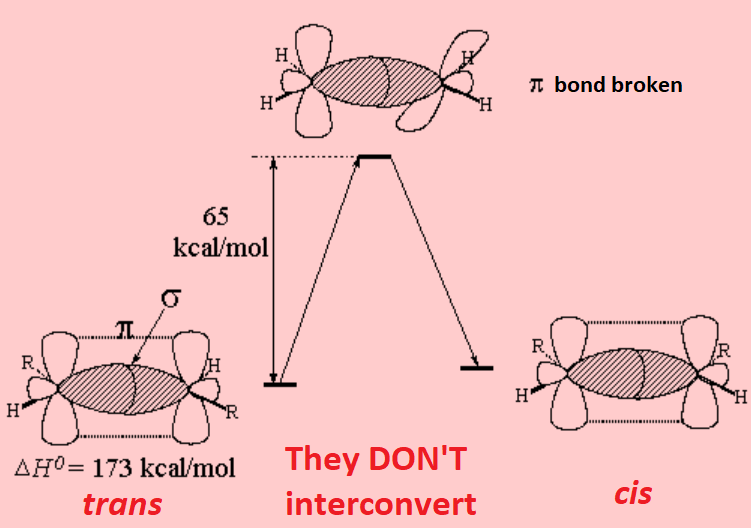
Rotation of a double C=C bond could be done but at a huge energy cost. Such a twisting involves the disruption of the lateral overlapping of the "p" orbitals that form the "pi" cloud of the double bond. That means about twenty times as much energy than that necessary to twiddle a single bond.
In addition to that fact, have you wondered whether there is an energy difference between cis/trans or E/Z isomers?
If you have and want besides to get an answer, the energy of hydrogenation of alkenes will give you an experimental answer.
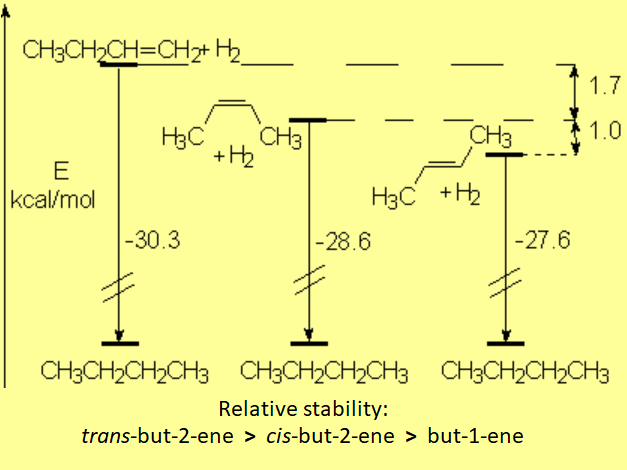
The energy released when alkenes are hydrogenated is a good measure of their relative stability:
Comparing the three possible butenes and their hydrogenation to the same product, butane, the trans isomer is the one releasing less energy. That means graphically that the trans isomer is the lowest in energy and thus the most stable one.
In contrast, but-1-ene is the least stable because it results in the most exothermic hydrogenation process of the three.
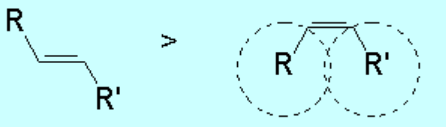
In general, olefins with the bulkiest groups in trans or E arrangement are more stable.
Besides, the stability of a double bond increases with substitution:
The relative higher stability of trans or E isomers, as compared to their cis or Z counterparts, can be easily understood on steric grounds as shown: