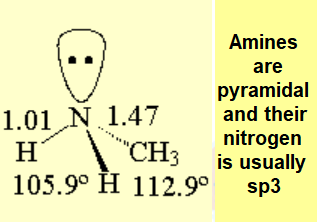
PHYSICAL PROPERTIES OF AMINES
In general boiling and melting points of amines are higher than those of the corresponding alkanes. Hydrogen bonding is important among the amine molecules at least having a N-H group
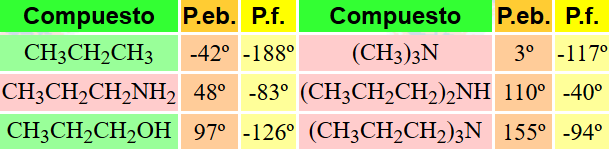 Secondary amines, and even tertiary ones where hydrogen bonding is not possible, have relatively high b.p. because of their size and the increase in van der Waals attraction among molecules.
Secondary amines, and even tertiary ones where hydrogen bonding is not possible, have relatively high b.p. because of their size and the increase in van der Waals attraction among molecules.
When alcohols and amines of similar molecular weight are compared, the latter display lower physical constants.
That means that the hydrogen N-H...N bonds are weaker than the
O-H...O ones.
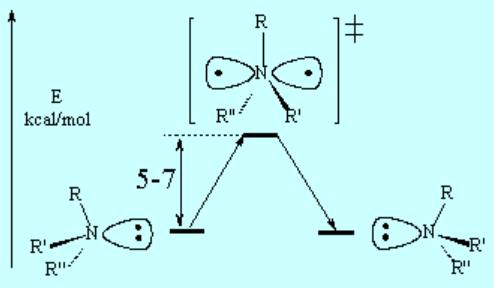
The mechanism of this inversion process goes through a TS where nitrogen is planar and sp2 hybridiced.
The energy barrier to nitrogen inversion is in general so low that the process happens for example in ammonia at a rate of 2·10^11 times per second! at room temperature.
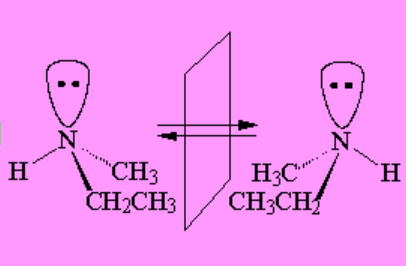
A consequence of this inversion is that, in general, amines are NOT configurationally stable.
That is to say, if object and image are not superimposable, the nitrogen inversion interconverts one another and, inevitably, racemization takes place.
| |
|---|
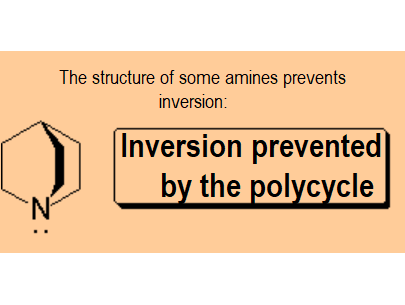
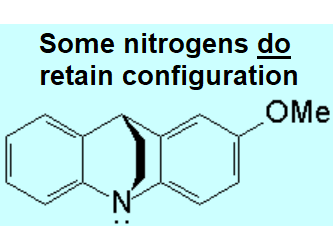
| Nitrogen cannot invert because it is included in a bicycle. | Nitrogen is a stereogenic center because it bears three different substituents plus the electron lone pair. It cannot invert and the molecule is chiral: there is a pair of enantiomers that in this particular structure cannot interconvert each other |
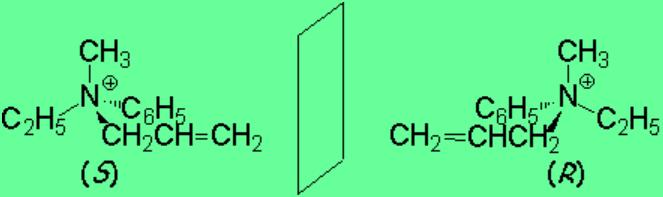
In ammonium salts, nitrogen is tetrasubstituted and, like a carbon, it maintains its configurational integrity and becomes a stereogenic center.
 Secondary amines, and even tertiary ones where hydrogen bonding is not possible, have relatively high b.p. because of their size and the increase in van der Waals attraction among molecules.
Secondary amines, and even tertiary ones where hydrogen bonding is not possible, have relatively high b.p. because of their size and the increase in van der Waals attraction among molecules.