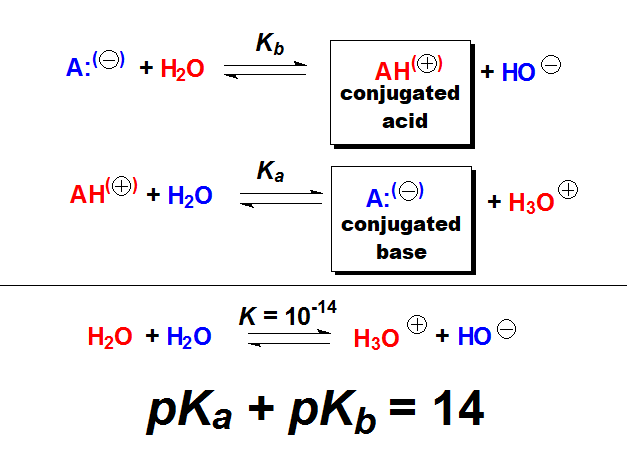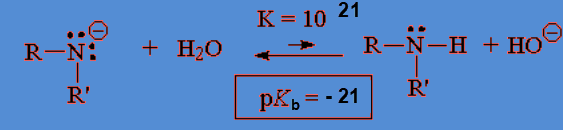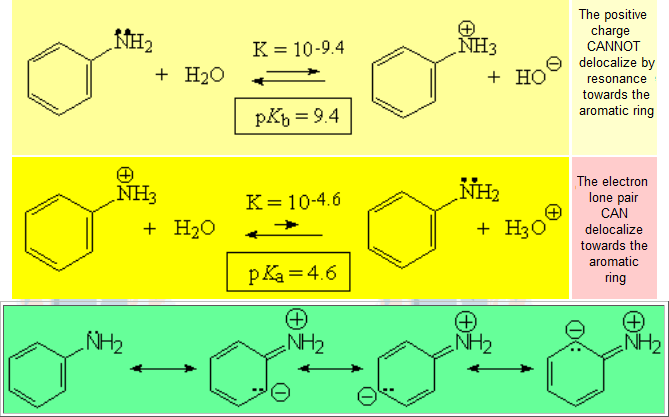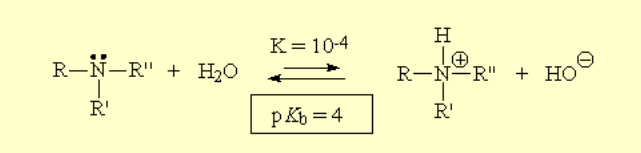
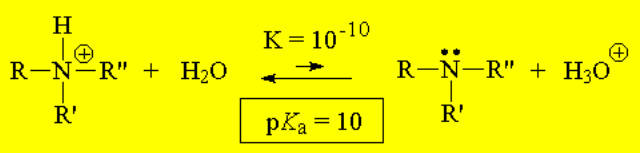
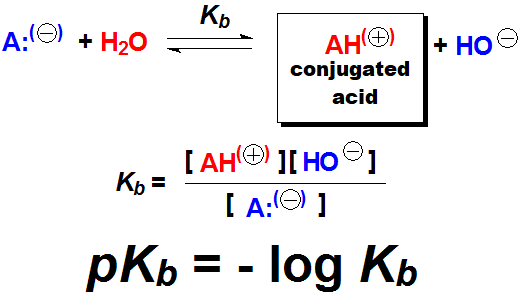 The higher the basicity, the more shifted the equilibrium to the right, i.e. to the formation of HO-, the higher Kb and the lower pKb.
The higher the basicity, the more shifted the equilibrium to the right, i.e. to the formation of HO-, the higher Kb and the lower pKb.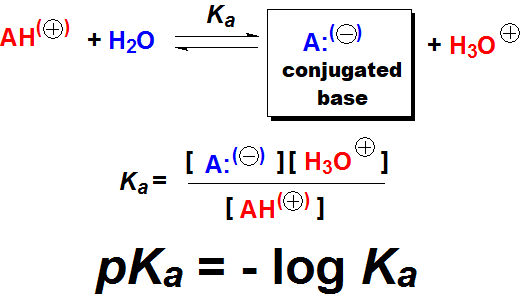 The higher the acidity, the more shifted the equilibrium to the right, i.e. to the formation of H+, the higher Ka and the lower pKa.
The higher the acidity, the more shifted the equilibrium to the right, i.e. to the formation of H+, the higher Ka and the lower pKa.