Crown ethers are perhaps the simplest molecules mimiking enzime bahavior, which is essential for life development.
Enzimes carry out very selective chemical transformations.
The very first step for an enzime to exert its biological function is to perform a extremely precise molecular recognition upon a given molecular shape - the substrate -, discarding other even pretty similar ones.
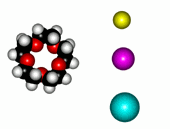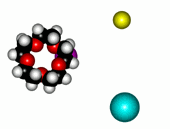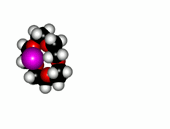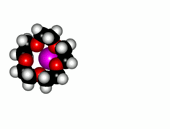
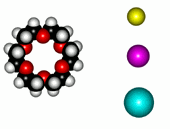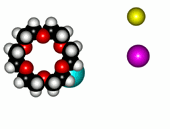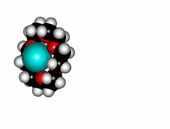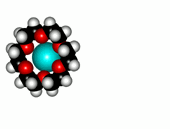
Crown ethers are able to selectively recognize alkaline metals due to the different size of their oxygen groove, that attracts by electrostatic forces the positively charged metal.
 |  |  |
|---|
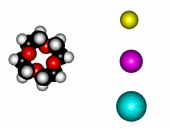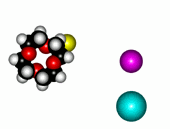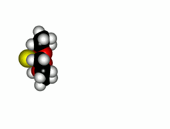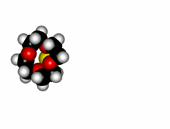
| 12-Crown-4/Li+ | 15-Crown-5/Na+ | Éter 18-Crown-6/K+ |
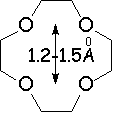 | 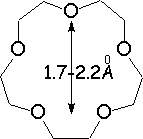 | 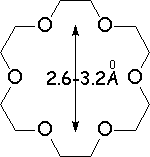 |
The alkaline metals Li+, Na+ and K+ have quite different ionic radii fitting best with a given crown ether.
In this way, by using different crown ethers, one could separate the alkaline ions that otherwise would be very difficult to get appart from each other.
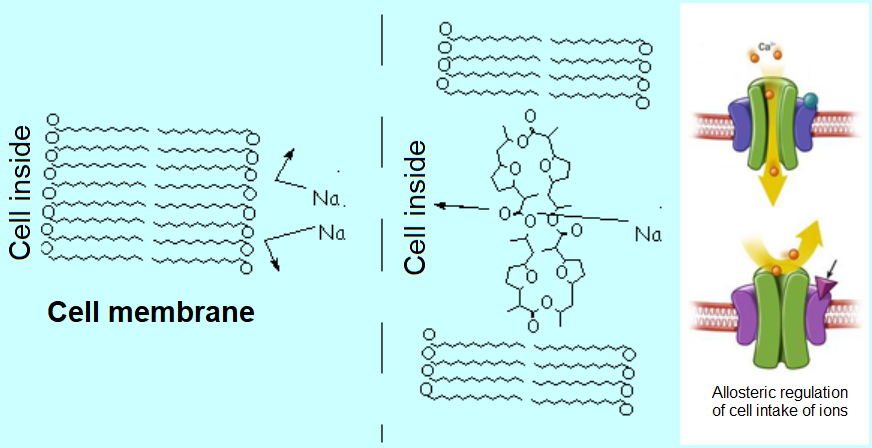
Sodium and calcium ions are essential for the living cells.
But these highly hydrophilic ions cannot get across the highly lipophilic cell membranes unless certain natural crown-ether-like compounds intercalate on them, thus allowing the selective entrance of these ions.
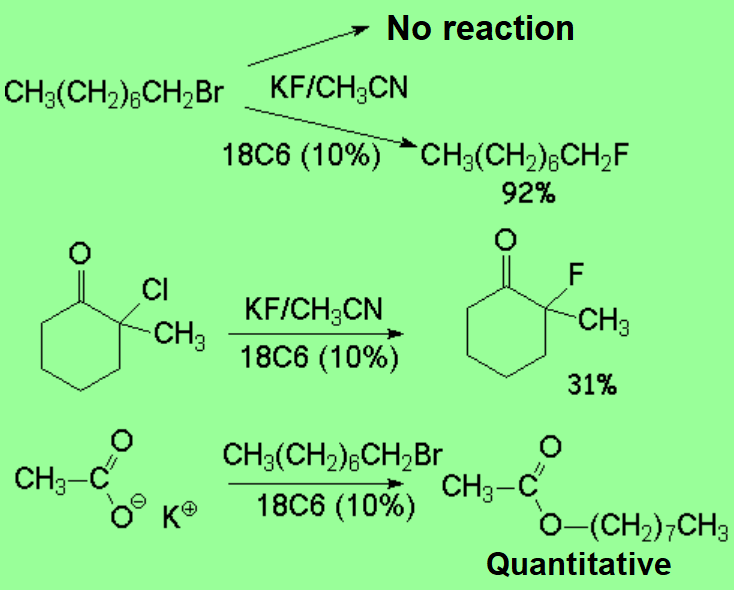
The chelation effect that crown ethers exert upon the alkaline ions induces an indirect consequence on the negative, nucleophilic ions that go with them.
By the use of crown ethers, one can increase the nucleophilicity of nucleophiles because the positive counterion is separated.
The presence of even a small amout of crown ether can make a reaction, that otherwise wouldn't take place, be possible.