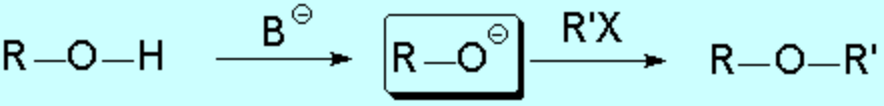
The simplest way of preparing ethers is the reaction between an alkoxide and an alkyl halide or sulfonate, following a typical SN2 sequence:
This reaction was discovered by Williamson at the end of the XIX century.
| | fig1 | fig2 | fig3 |
|---|
Typical
solvents
| The same ROH
in excess | | | |
| | | Dimethylsulfoxide
(DMSO) | N,N-dimethyl
formamide (DMF) | Hexamethyl
phosphoramide
(HMPA) |
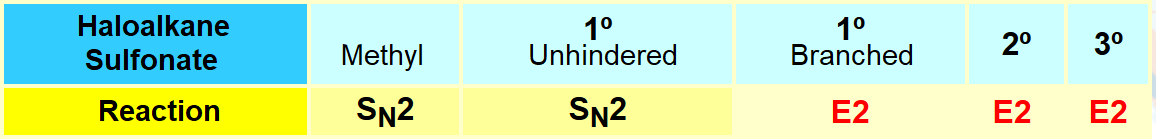
However, the usefulness of this reaction is heavily limited by the strong basicity of the alkoxide:
Elimination is therefore a strong competitor to this reaction.
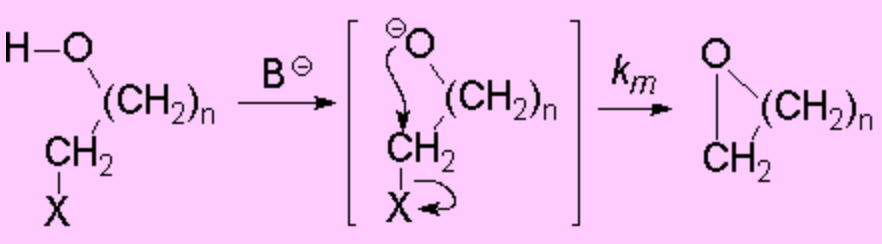
Should the Williamson process be intramolecularly carried out, one obtains cyclic ethers
(Intramolecular SN2 reaction).
Depending on the ring size that is being formed (m = n+2), the reaction proceeds at different rates:
For the ring formation to take place, both ends of the molecule must encounter and collide.
The higher 'n', the larger the number of bonds between the alkoxide and the leaving group.
Those bonds have to be pretty ordered for the molecule ends to collide.
This adds an unfavorable enthropic term to the reaction rate.
In the case of the epoxide this enthropic contribution is the lowest.
That's why the three-membered ring is the fastest to be formed, despite the ring tension in the final product.
Stereochemistry and conformation are essential for a reaction to proceed or not.
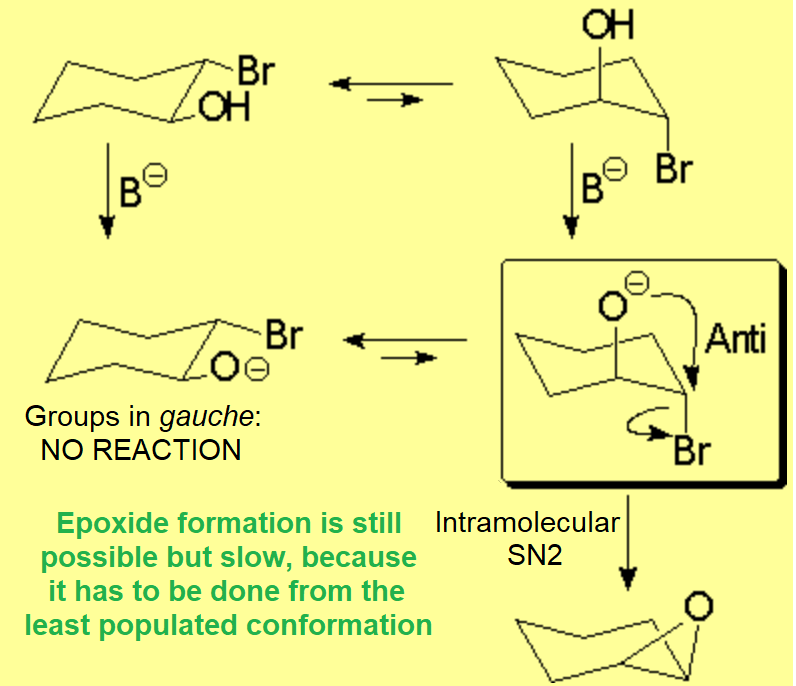 The trans isomer of 2-bromo cyclohexanol can lead to the epoxide, even though the right conformer is not the most populated - stable - one.
The trans isomer of 2-bromo cyclohexanol can lead to the epoxide, even though the right conformer is not the most populated - stable - one.
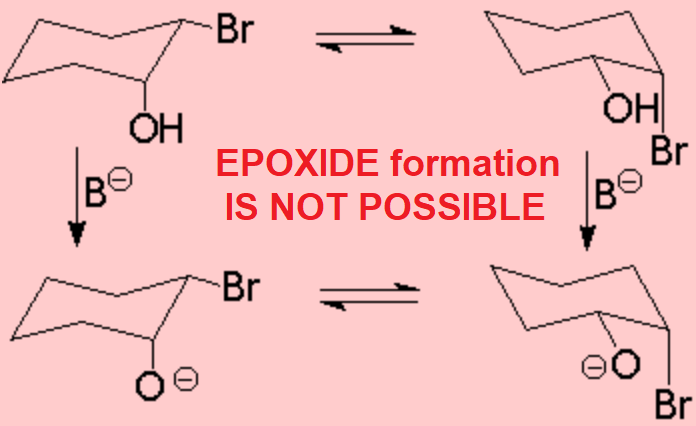 In turn, the cis isomer of 2-bromo cyclohexanol does not have any conformer with the right arrangement for the alkoxide to attack, by the opposite side, the carbon bearing the leaving group.
In turn, the cis isomer of 2-bromo cyclohexanol does not have any conformer with the right arrangement for the alkoxide to attack, by the opposite side, the carbon bearing the leaving group.
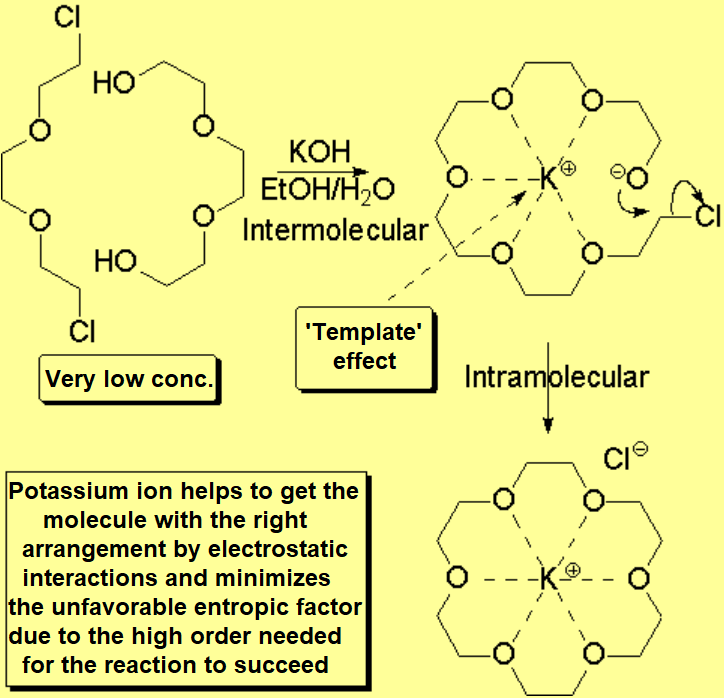
The so-called crown ethers, subjected to the Noble Prize, can be synthetized taking advantage of what has been called 'template effect'.

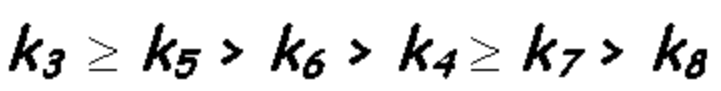
 The trans isomer of 2-bromo cyclohexanol can lead to the epoxide, even though the right conformer is not the most populated - stable - one.
The trans isomer of 2-bromo cyclohexanol can lead to the epoxide, even though the right conformer is not the most populated - stable - one. In turn, the cis isomer of 2-bromo cyclohexanol does not have any conformer with the right arrangement for the alkoxide to attack, by the opposite side, the carbon bearing the leaving group.
In turn, the cis isomer of 2-bromo cyclohexanol does not have any conformer with the right arrangement for the alkoxide to attack, by the opposite side, the carbon bearing the leaving group.