Structurally speaking, ethers can be considered as derived from water or alcohols, in which the two or one hydrogens have been respectively replaced by carbon chains.
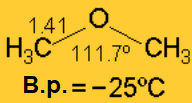
The angular structure of ethers is well explained by assuming that the oxygen is sp3 hybridized and bears two electron lone pairs.
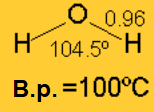
Ethers cannot establish hydrogen bonding and hence, they display much lower boiling and melting points that the refereable alcohols.
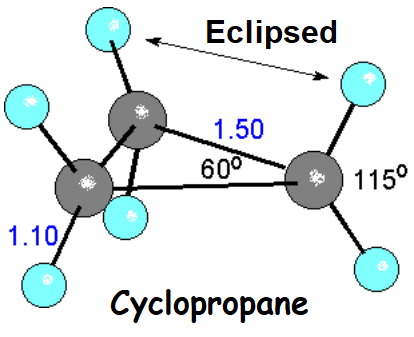
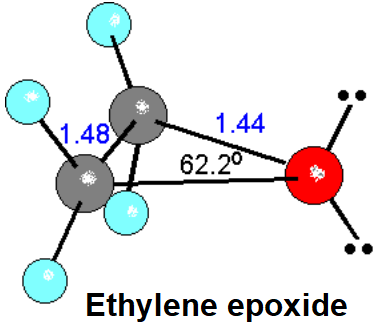
A very special and important case is that of the epoxides - oxyranes - , the three-membered cyclic ethers.
The ring contains a high tension, similar to that of cyclopropane.
The tension and the oxygen makes it be very reactive.
 |
|---|
|
| Ethylene oxide |