PREPARATION OF ALKENES: ELIMINATION
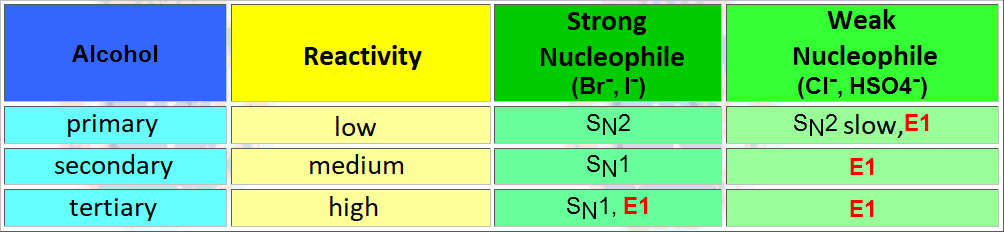
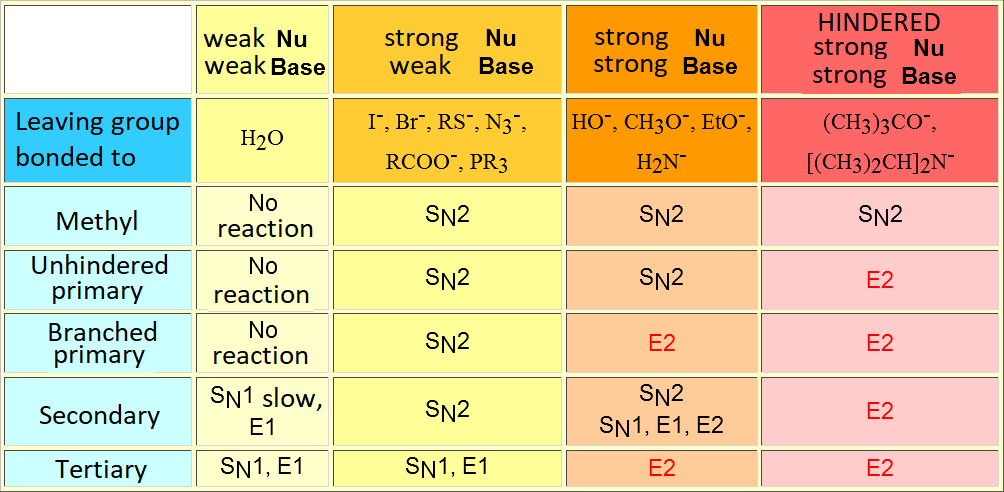
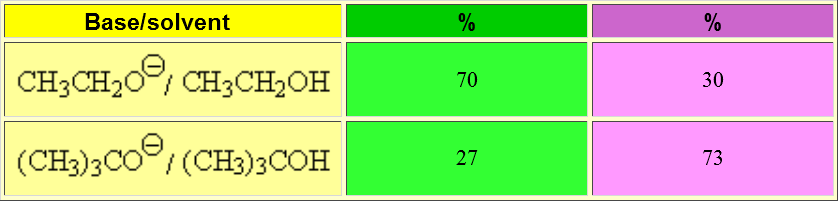
An elimination reaction is promoted by a base and is always in competition with substitution. The ratio of elimination can be maximized by using strong, heavily hindered bases.
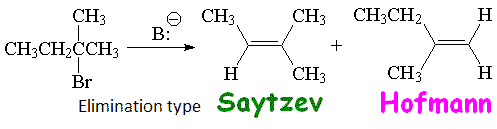
The bulkiest base has not many attack choices but the methyl hydrogens which are the least hindered ones. That leads to a higher ratio of the thermodynamically least stable olefin.
This is a clear-cut case of kinetic vs. thermodynamic control.
Let's recall the amusing story...
 Aleksandr Mijáilovich Záitsev
Aleksandr Mijáilovich Záitsev
Aleksandr Mijáilovich Záitsev (también escrito como Zaytsev, Saytzeff y Saytzev; 2 de julio de 1841 – 1 de septiembre de 1910) fue un químico ruso. Trabajó en compuestos orgánicos y propuso la regla de Saytzev, que pronostica la composición de los productos de una reacción de eliminación.
August Wilhelm von Hofmann (8 de abril de 1818 – 5 de mayo de 1892) fue un químico alemán.
 August Wilhelm von Hofmann
August Wilhelm von Hofmann
Remember that elimination through an E2 mechanism requires that the leaving group and at least one hydrogen in the neighboring carbon be in anti arrangement.
This stringent stereoelectronic requirement make some reactions be stereospecific:
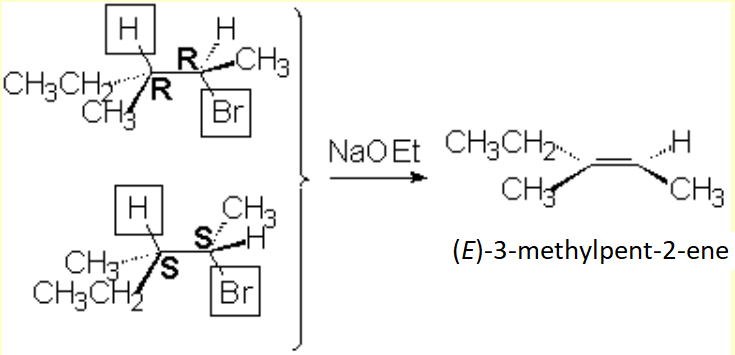 The 2R,3R and/or 2S,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the E isomer del 3-methyl-2-pentene.
The 2R,3R and/or 2S,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the E isomer del 3-methyl-2-pentene.
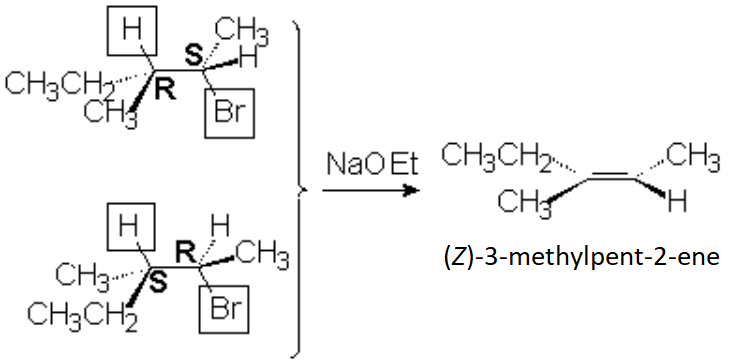 The 2S,3R and/or 2R,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the Z isomer del 3-methyl-2-pentene.
The 2S,3R and/or 2R,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the Z isomer del 3-methyl-2-pentene.
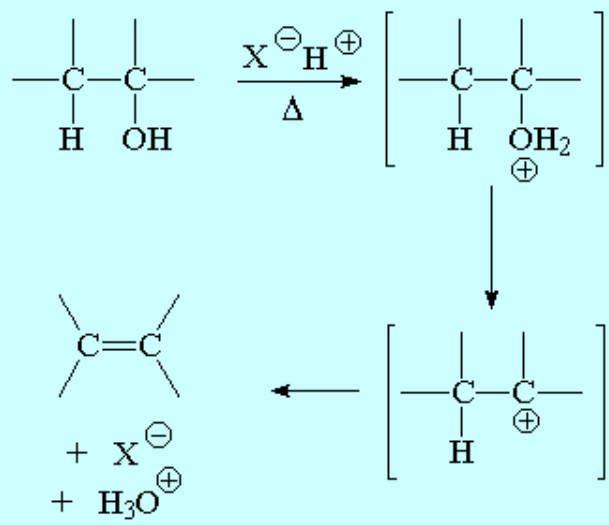
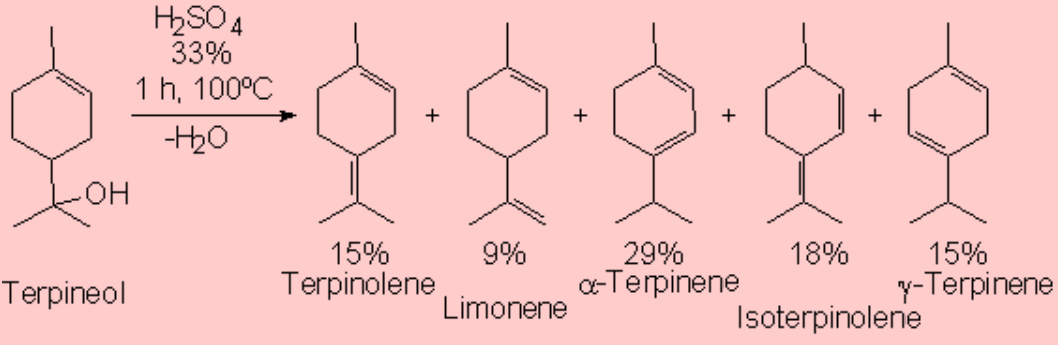
The treatment of an alcohol with a mineral acid at high temperature leads to water elimination (dehydration) and olefin formation.
The reaction proceeds by an E1 mechanism and hence through carbocations.
As usual, carbocations may suffer rearrangement and yield unexpected olefins.

 Aleksandr Mijáilovich Záitsev
Aleksandr Mijáilovich Záitsev August Wilhelm von Hofmann
August Wilhelm von Hofmann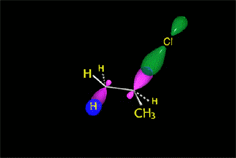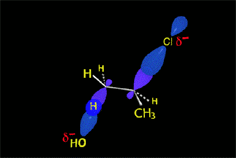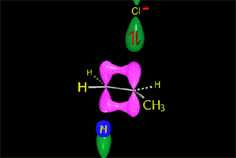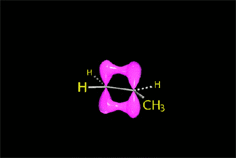
 The 2R,3R and/or 2S,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the E isomer del 3-methyl-2-pentene.
The 2R,3R and/or 2S,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the E isomer del 3-methyl-2-pentene. The 2S,3R and/or 2R,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the Z isomer del 3-methyl-2-pentene.
The 2S,3R and/or 2R,3S isomers (together -racemate- or individually) of 2-bromo-3-methylpentane by E2 elimination lead exclusively, stereospecifically, to the Z isomer del 3-methyl-2-pentene.