HYDROGEN HALIDE ADDITION TO ALKENES
Hydrogen halides easily undergo heterolytic cleavage.
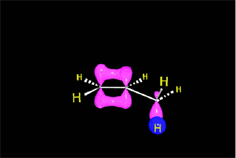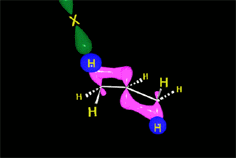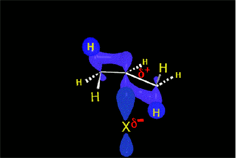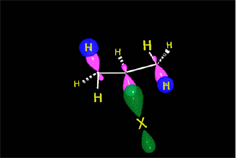
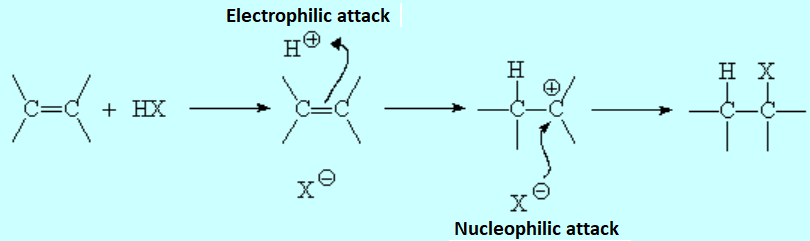
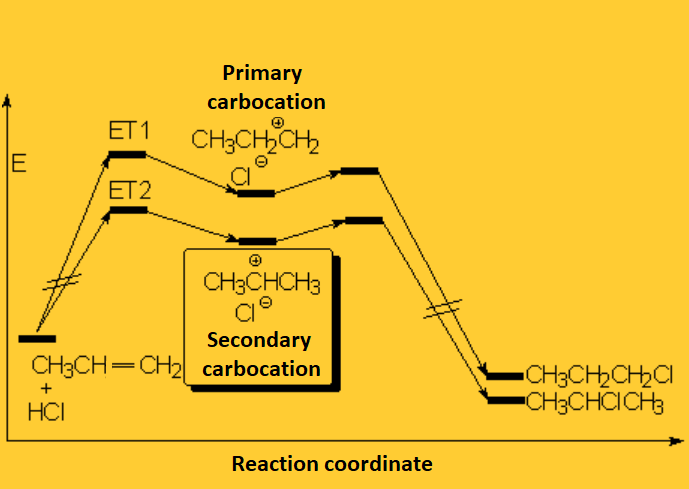
The resulting proton is quite strong a electrophile and attacks the 'pi' cloud of a doble C=C bond, leading to a carbocation (the least unstable!!!) that colapses with the halide counterion.
Any of the four hydrogen halides (HI, HBr, HCl or HF) gives the addition reaction in a regioselective way:
The formed product depends on the relative stability of the possible intermediate carbocations.
In short, the initial protonation produces the least unstable carbocation.
Long before chemists knew what a carbocation is, Markovnikov studied the reaction on a large series of olefins and stablished his famous rule.
Markovnikov's rule: The proton of the hydrogen halide bonds to the least substituted carbon.
 Vladimir Vasilyevich Markovnikov , also spelled as Markownikoff, (December 22, 1838 – February 11, 1904), was a Russian chemist.
Vladimir Vasilyevich Markovnikov , also spelled as Markownikoff, (December 22, 1838 – February 11, 1904), was a Russian chemist.
But, what happens if we add a peroxide to the reaction medium?
The regiochemistry of the reaction changes.
The mechanism of the reaction should thus be different.
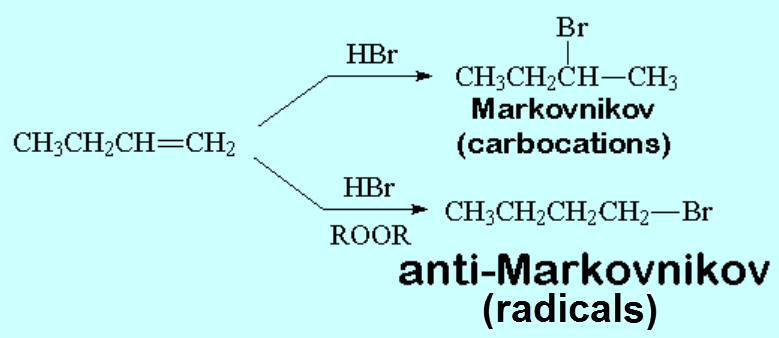
Consider now that the attacking species is the radical halide. Do you understand why the regiochemistry changes?
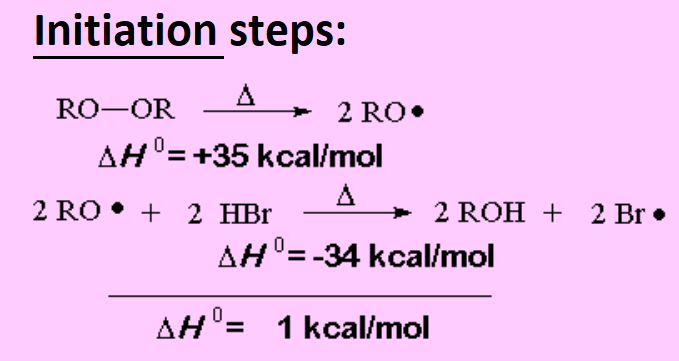
The peroxide makes the mechanism proceed through radicals instead of carbocations:
The peroxide homolytically splits into two oxyalkyl radicals that quickly react abstracting the hydrogen from HBr and forming the Br· radical.
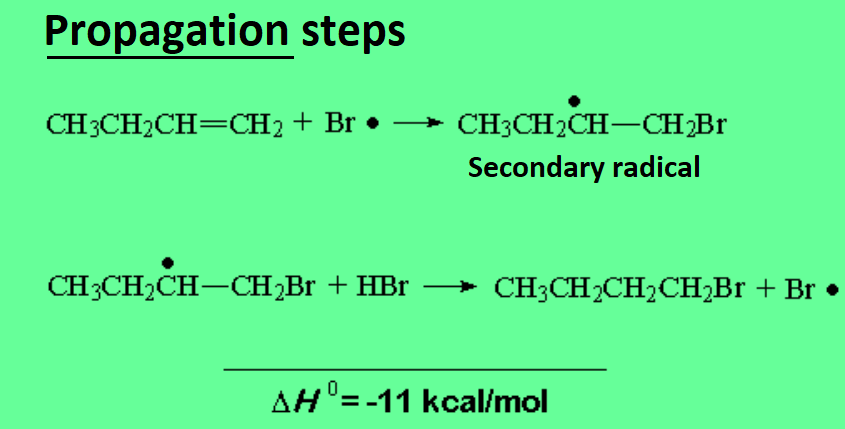
Now Br·, NOT H+!, attacks the olefin rendering the least unstable secondary C· radical.
This is the key to the different regiochemistry:
Without peroxides the reaction proceeds through carbocations;
with peroxides the attacking species is Br· and the reaction goes via radicals.
In both cases the reaction patway is that of the lowest possible energy.
 Vladimir Vasilyevich Markovnikov , also spelled as Markownikoff, (December 22, 1838 – February 11, 1904), was a Russian chemist.
Vladimir Vasilyevich Markovnikov , also spelled as Markownikoff, (December 22, 1838 – February 11, 1904), was a Russian chemist.