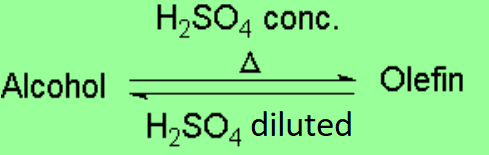WATER ADDITION TO ALKENES
The addition of water to a double C=C bond yields an alcohol, being the opposite reaction to the dehydration of the latter.
The electrophilic addition of water to olefins is thus reversible. The sense of the reaction depends on the amount of water in the medium.
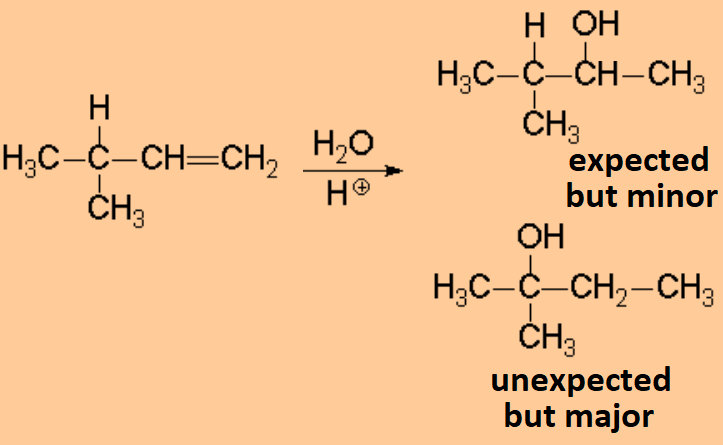
The addition of water to olefins is carried out in acidic medium and the reaction proceeds through carbocations. As it is usual when carbocations are involved, the major product might be unexpected.
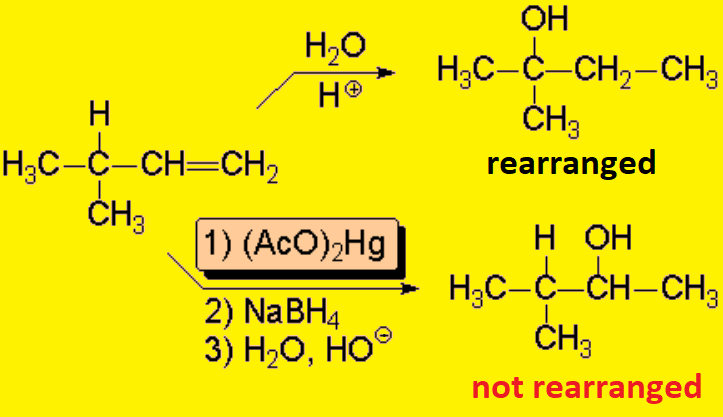
There exists an extremely interesting reaction that renders the formal addition of water to olefins but avoiding carbocations:
The Oxymercuration-Demercuration:
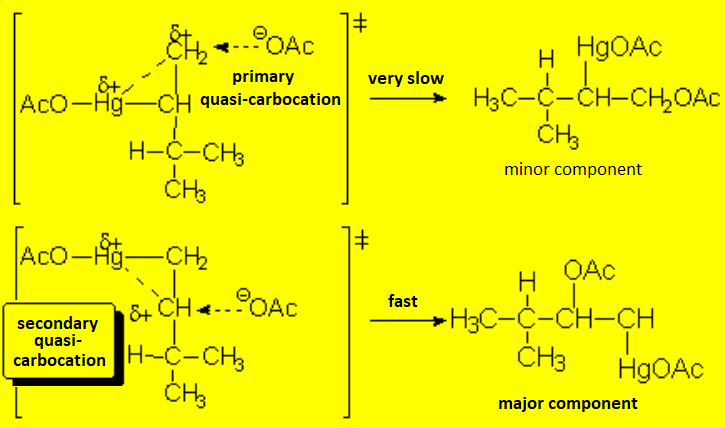
The mechanism reminds of the aperture of a bromonium ion or that of a protonated epoxide. The true carbocation is never formed and rearrangements are hence avoided.
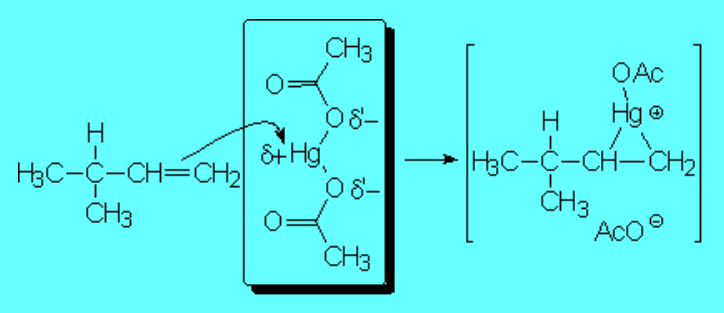
The first step is the attack of the "pi" cloud to the metal, very electron deficient, leading to an organometalic three-membered cyclic compound of mercury
The formed organomercurial reminds of a bromonium ion or a protonated epoxide, bears a lot of tension and opens up following suit, i.e. leading to the least unstable quasi-carbocation by a SN1-like mechanism.
Finaly, sodium borohydride breaks up the C-Hg bond and the metal is taken over by a hydrogen. The base saponifies (hydrolizes) the acetate ester to render the alcohol.