INTERPRETING MULTIPLE-BOND 2D H/C
CORRELATION
(HMBC)
It's already some 30 years since the bidimensional techniques revolutionized structural elucidation by NMR. The theory behind the recording of these spectra exceeds the goals of this course. We will however study their interpretation which is fortunately much easier.
1H and 13C display long-range correlations, i.e. through two or three bonds in the so-called HMBC spectrum, acronym of Heteronuclear Multiple Bond Correlation.
Less names and more examples!!!
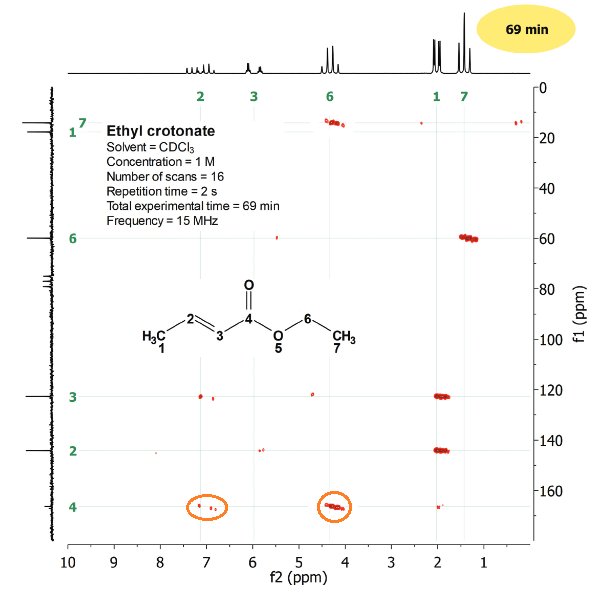
Look at the multiple-bond 2D H/C correlation of ethyl crotonate. The compound's structure in on the figure to help you understand how these spectra are interpreted.
To be honest, the utility of these spectra is to determine unknown structures but we cannot start building the house from the roof...
Please, make a list of the first features you just see:
1) There are many correlations within the paralelogram.
2) In the upper part there is a conventional 1D 1H-NMR spectrum. 3) In the left hand side there is a regular 1D 13C-NMR spectrum.
4) The vertical and horizontal scales are in ppm and typical of 13C- and 1H-NMR spectra, respectively.
4) Many of the protons and carbons show each more than one correlation.
5) The non-hydrogenated carbons, like the carbonyl group, display correlations.
Before our start deciphering the 2D spectrum, let's understand the 1D 1H- and 13C-NMR spectra.
1H-NMR:
1) The signals at 1.3 ppm (triplet) and 4.2 ppm (quartet) belong to the CH3CH2 group.
2) By applying the N+1 one understands that the CH3 shows up as a triplet (N = 2 of the neighboring CH2) and the CH2 as a quartet (N = 3 of the neighboring CH3).
3) The signal at 2.0 ppm (double-doublet) belongs to the CH3 linked to the double bond.
4) The latter CH3 couples to each of the two olefin 1H but through a different coupling constant because the distance to each of them is differen: C(1)H3-C(2)H-C(3), 3 bonds with HC(2) and 4 bonds with HC(3).
5) The signals at 6 ppm y 7.2 ppm belong to the olefin protons and they are very complex because they are coupled one another and with the CH3 group.
6) Both of the latter signals are "double-quartets": quartet due to the coupling to CH3 (N = 3) and double due to the coupling with the other olefin H (N = 1).
7) The quartets' four lines of the signal at 6 ppm are closer (lower coupling constant) to each other than in the signal at 7.2 ppm suggesting that the signal at 6 ppm belongs to the olefin H(C3) farther away from C(1)H3. The larger the distance, the lower the coupling constant.
13C-NMR:
1) There are three signals between 10 and 60 ppm that might belong to the aliphatic carbons but we cannot know which is which. Maybe the signal close to 60 ppm belongs to the carbon bonded to the electronegative oxygen.
2) The two signals between 120-140 ppm might belong to the olefin carbons but we don't know which is which either.
3) The most deshielded signal at 165 ppm should belong to the carbonyl carbon due to its high chemical shift and low intensity, the latter typical of non-hydrogenated carbons.
Let's get into the multiple-bond 2D H/C interpretation, in comparison with the corresponding single-bond 2D H/C:
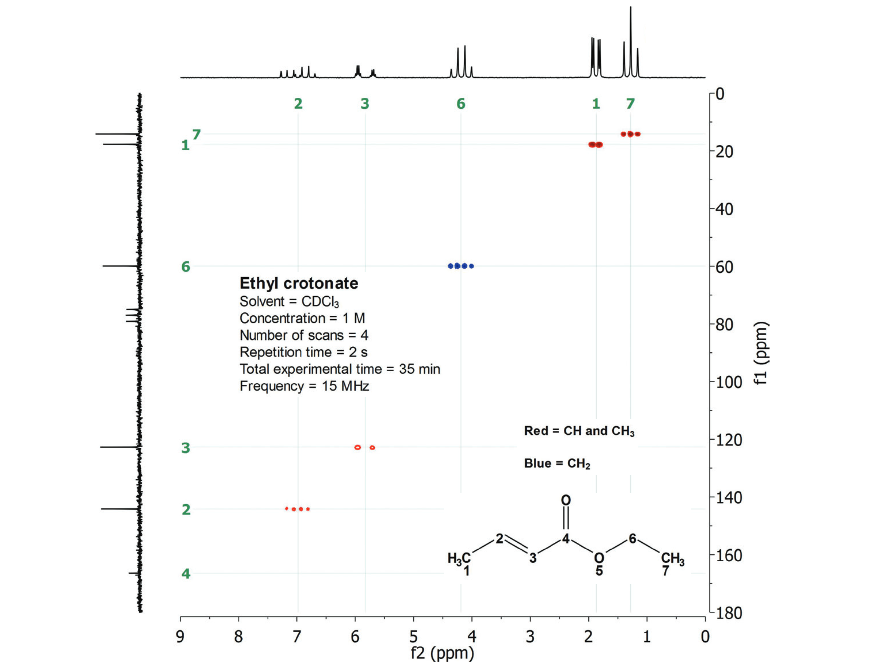 Single-bond 2D H/C
Single-bond 2D H/C
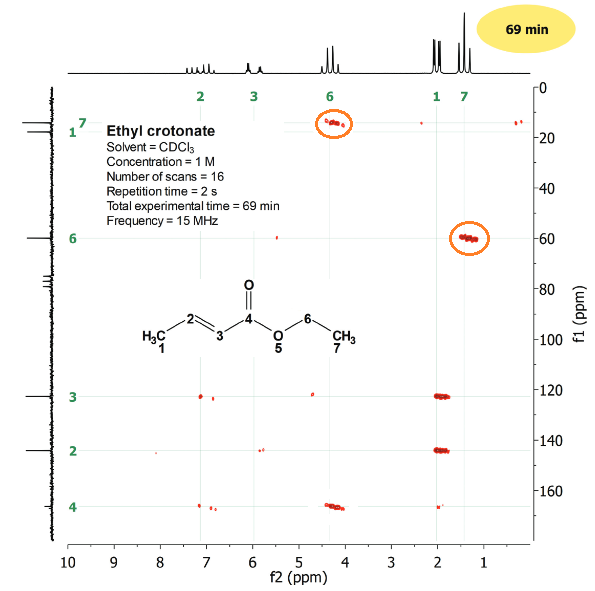 Multiple-bond 2D H/C
Multiple-bond 2D H/C
We know before hand that the CH3CH2 group displays 1H signals at 1.3 ppm and 4.2 ppm, each of them correlating with its directly bonded carbon.
The single-bond pairings are:
1.3 ppm/14 ppm and 4.2 ppm/60 ppm.
Multiple-bond CH3CH2 correlations should obviously inverted:
The multiple-bond pairings are:
1.3 ppm/60 ppm and 4.2 ppm/14 ppm.
Now the CH3 group protons (1.3 ppm) correlate with the CH2 carbon (60 ppm) at two-bond distance and the CH2 protons (4.2 ppm) do with the CH3 carbon (14 ppm), at a distance of two bonds as well.
It is key to realize that the carbonyl carbon does display correlations.
It correlates with the CH2 protons on one hand and with the olefin CH proton on the other, all of them at three-bond distance.
The carbonyl carbon can be securely located in the molecule due to these correlations.
Reflect on this: it is difficult to find non-hydrogenated carbons, i.e. carbons without directly bonded hydrogens, not having hydrogen(s) at neighboring positions two or three bonds apart.
The multiple-bond 2D H/C correlation allows us to place the non-hydrogenated carbons in an unknown structure.
Was the interpretation easy? A piece of cake!!!
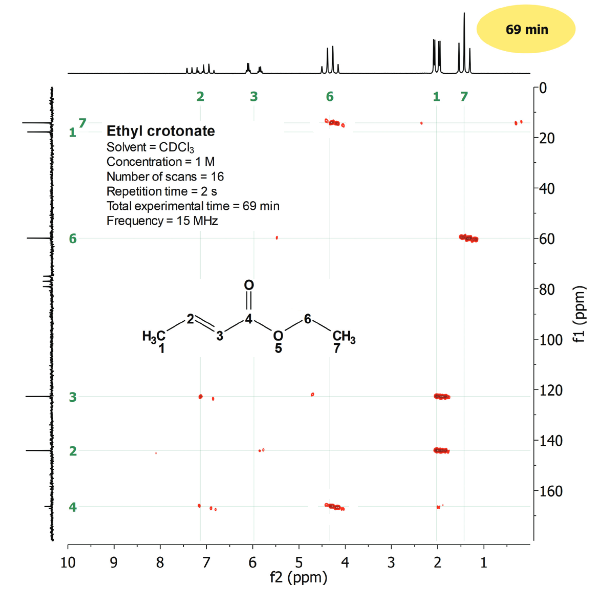
The multiple-bond 2D H/C correlation led us to the completion of the structure.
We now have all tools at our disposal to our smart determination of unknown structures.
We'll see several examples...
 Single-bond 2D H/C
Single-bond 2D H/C
 Multiple-bond 2D H/C
Multiple-bond 2D H/C