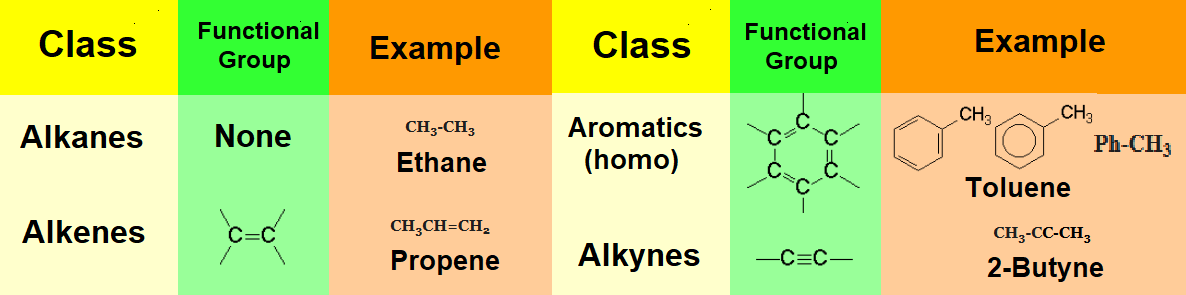
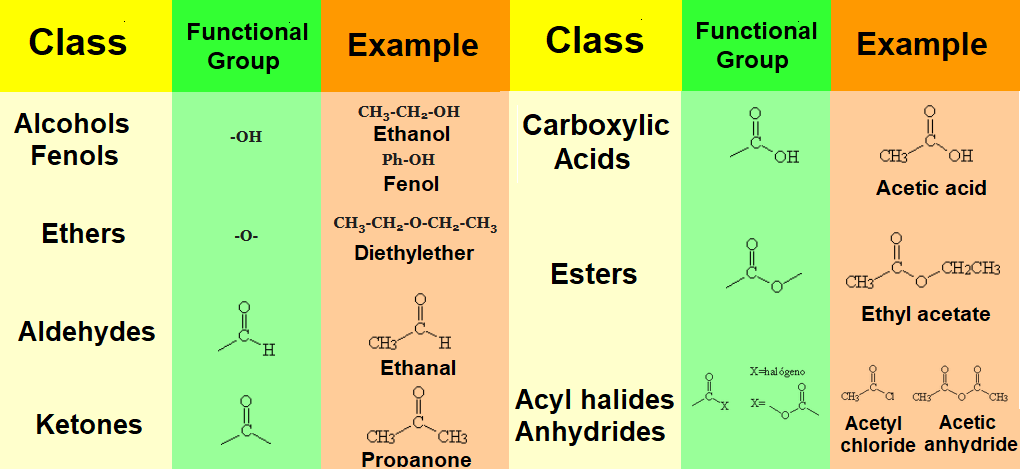
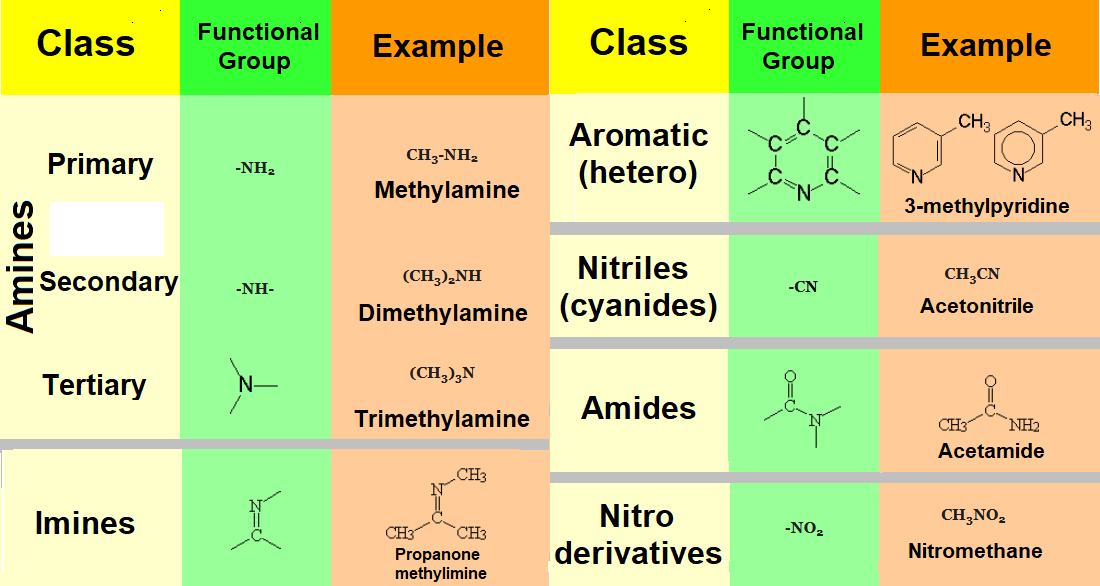
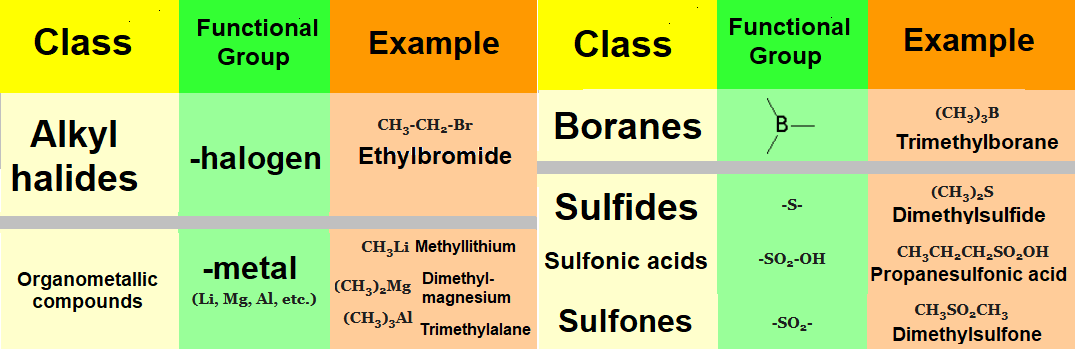
Organic molecules consist of a hydrocarbon chain to which some special atomic arrays insert or bind.
This 'special' arrangement of atoms, almost always different from carbon and hydrogen ('heteroatoms'), are called functional groups.
One of the first tasks in your tackling with Organic Chemistry is the recognition and identification of the diverse functional groups present in a given molecule.
Click in 'Molecular panel' available across all pages and click 'FG' within ('Functional groups')
You'll get a list of several functional groups.
Click on one of them, then on the blank panel and finally on the arrow pointing to the black panel.
You'll be presented with the 3D structure of the selected functional group.
Draw a hydrocarbon chain and attach functional groups to it.
Look at the below list, try to build your own functional groups based on it and display them in 3D projections.
Groups WITHOUT heteroatoms
Groups with other heteroatoms
Once the diferent functional groups have been identified, one of them becomes the main function giving its name to the molecule.
The simplified order in which functional groups take the lead over the molecule name is:
1.- Acids
(carboxylic > sulfonic)
2.- Acid derivatives
(anhídridos > ésteres > haluros de acilo > amidas > nitrilos)
3.- Aldehydes > ketones
4.- Alcohols > phenols > thiols
5.- Amines
6.- Ethers > thioethers
7.- Aromatics > Alkenes > alkynes
The main function determines:
1.- The compound's name.
2.- The main hydrocarbon chain that must be the longest possible containing the main function.
3.- The localizing numbers of substituents and/or secondary functions.
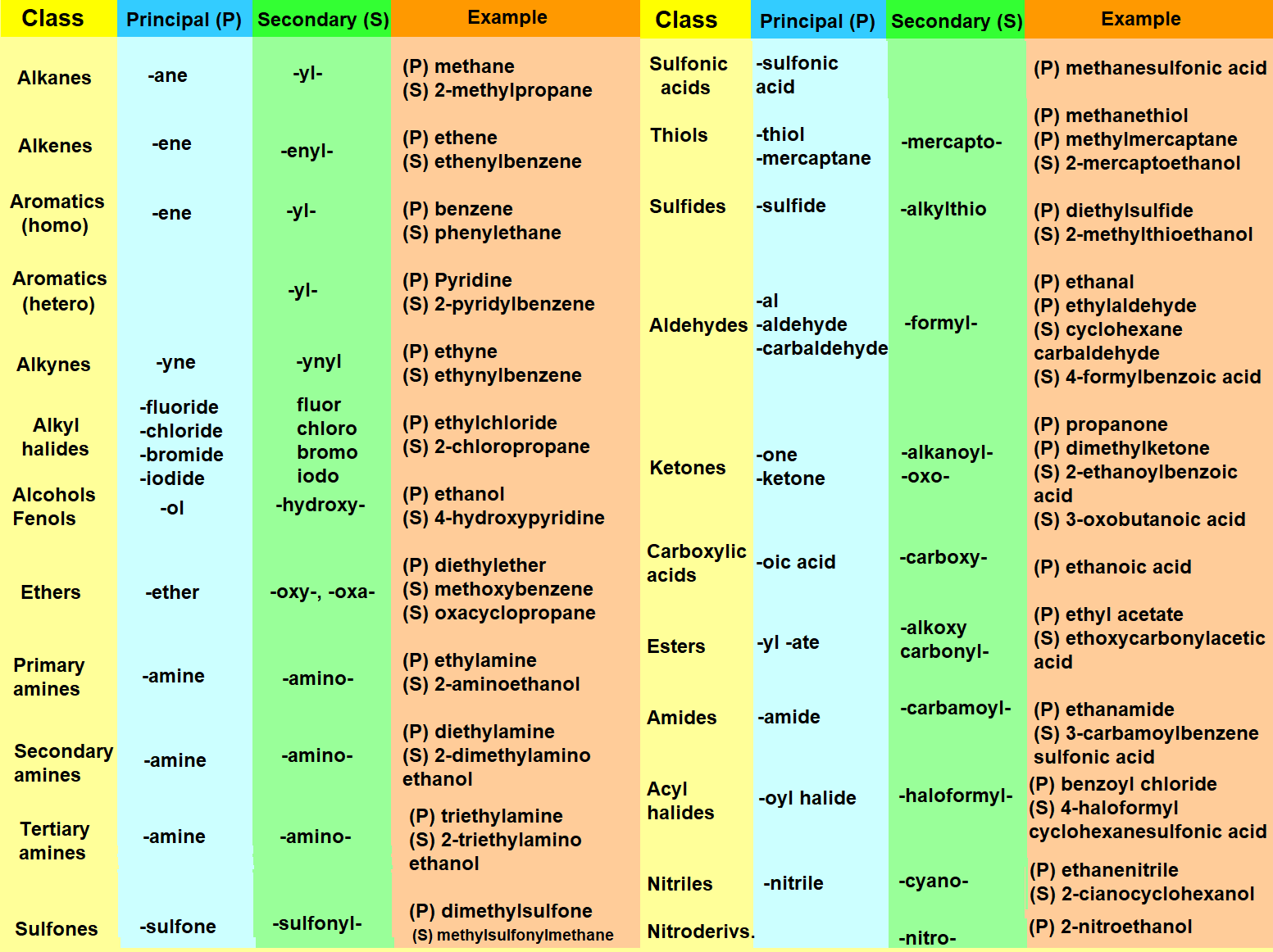
Nomenclature of the most common functional groups when they are principal (P) or secondary (S):