Robert S. Cahn (1899-1981): Member of the Royal Institute of Chemistry of London.
Christopher Ingold (1893-1970): Chemistry Professor of the University College of London.
Vladimir Prelog (1906-1998): Former Yugoslavian Chemist, Swiss nationalized.
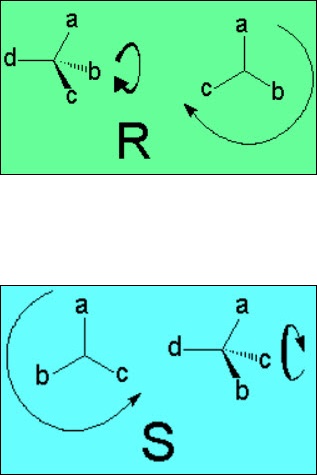
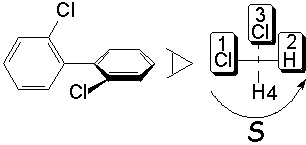
By definition, the group with the least priority (d) is left pointing backwards. One then goes turning around from the highest priority group (a) to the least one (c) of the remaining three groups.
If the turning is clockwise, configuration is R (rectus).
Otherwise it is S (sinister).
PRELATION (PRIORITY) RULES
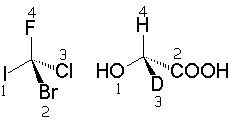
The atomic weight of the atoms (isotopes) directly bonded to the stereogenic element determines its priority.
The atom with the highest atomic weight is the one with the highest priority.
Hydrogen is obviously always the atom with the least priority, provided there is one attached to the stereogenic element.
If there is a 'tie', i.e. there are two equal atoms bonded to a stereogenic element, one must look at the next position to break the 'tie'. In the case of a persisten 'tie', look at the next posistion and so on and so forth until the 'tie' is broken.
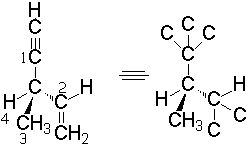
If some of the atoms bonded to the stereogenic element is involved in a multiple bond, the rule is that a double bond is like the carbon is formaly bonded to two atoms, and a triple like bonded to three.
That's a clever way to break 'ties' like that of the example.
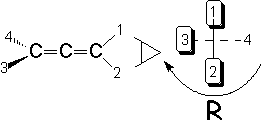
The R/S configuration can be also defined in molecules without stereocenters, like allenes for instance, but with stereogenic elements.
We first give to each substituent the priority in the same terms as above (atomic weight). We take the one with the least priority and put it pointing backwards. In this case there could be 'ties'. Just take one of them.
Look first at the front part of the molecule and assign the two substituents their priority (1 and 2). Look then at the back part of the molecule and do the same (3 and 4).
The clockwise or counter-clockwise turning to go from 1 to 2 and 3 gives us the R or S configuration, respectively.