In Organic Chemistry - the chemistry of carbon - hydrogen is also a very abundant element. Therefore, in the organic molecules there are numerous functions bearing hydrogens that can release protons and display ACIDITY properties.
We should first consider how a bond must break for the molecule to release a proton (H+).
HOMOLYTIC cleavage:
The two bond electrons are split between the two bonded atoms.
This way of breaking a bond is in general very difficult and only happens when the hydrogen is attached to an atom of low electronegativity.
HETEROLYTIC cleavage:
The two electrons are kept by the atom of the highest electronegativity.
This is the most common way of breaking a bond and happens when the hydrogen is attached to an atom of high electronegativity.
Functional groups bearing an O-H group may suffer heterolytic cleavage and display more o less powerful acidity properties.
In some cases, actually very important ones, the C-H bonds can be heterolytically broken and show acidity properties.
See some examples of acidity in organic molecules:
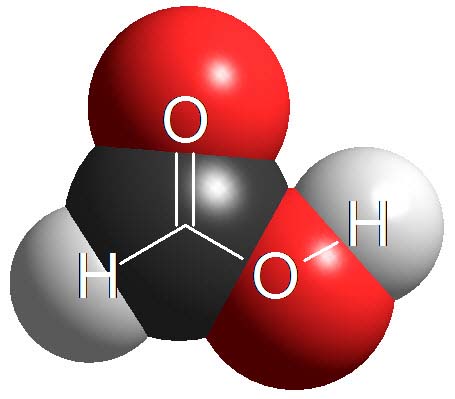 Carboxylic Acids
Carboxylic Acids
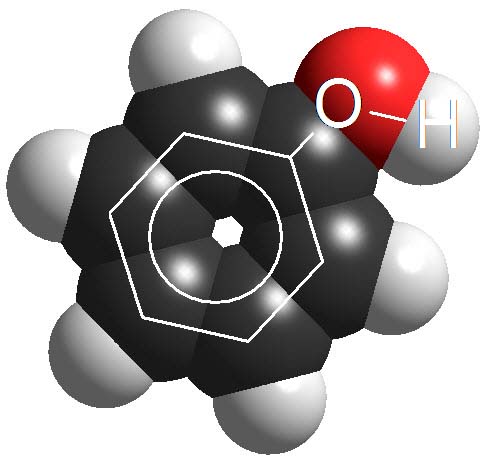 Phenols
Phenols
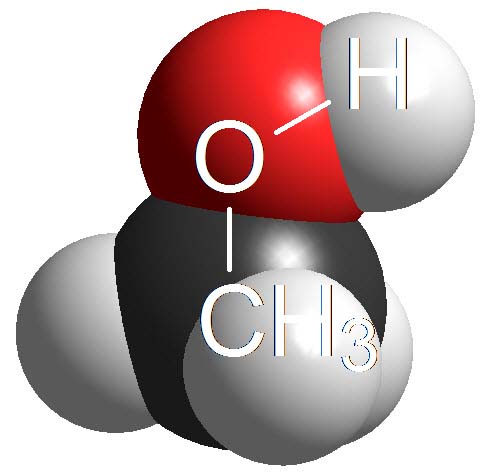 Alcohols
Alcohols
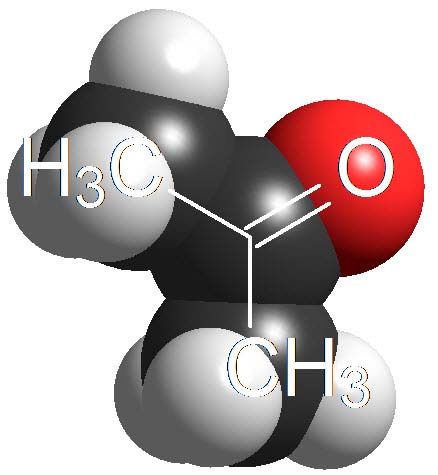 Ketones
Ketones
(Alpha C-H)
The assertion 'more or less powerful acidity properties' is vague and of low scientific value.
Is there any method to measure acidity and give it a number?
The pKa value is the numeric measurement of acidity and is related to the shift of the acid-base equilibrium of a compound with water.
Which are the pKa values of the aforementioned types of organic compounds?
In each kind there is a variable range of pKa values depending on each single structure. We will finely address this problem when studying the particular functional groups but, for the time being, let's give pKa's an introductory touch.
Carboxylic acids are surnamed 'acids' because they have the lowest pKa values (1-5) in organic chemistry. They are definitely the most acidic of the four kinds of compounds cited above.
However, there are stronger acids in chemistry, for instance inorganic sulfuric acid with a pKa = -3 (negative!).
How many times is sulfuric acid stronger than acetic acid (pKa = 4.9)?
Wrong! It's not 7.9 times but 10 to 7.9 times. Remember that the definition of pKa a logarithm is involved!!!
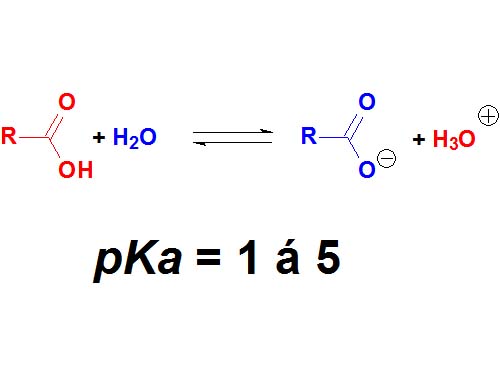 Carboxylic Acids
Carboxylic Acids
Phenols are not surnamed 'acids' because their pKa values are higher (7-10). They display acidic properties but their acidity is feeble.
How many times a phenol is less acidic than a carboxylic acid as an average?
Wrong again!!! It's not around 6 times but 10 to 6 times.
Remember once more that the definition of pKa involves a logarithm!!!
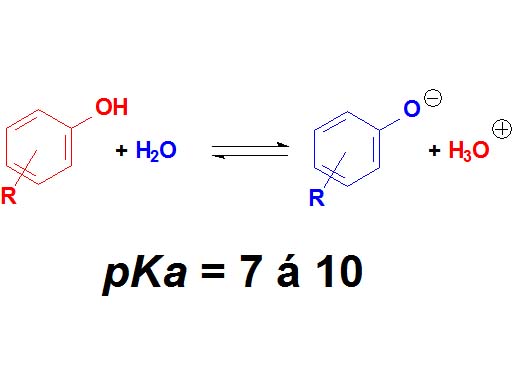 Phenols
Phenols
Alcohols are not either surnamed 'acids' because their pKa values are even higher (11-15). They display acidic properties but their acidity is even feebler than that of phenols.
How many times is an alcohol less acidic than a phenol as an average? Wrong again!!! It's not around 4 times but 10 to 4 times. Remember once more that the definition of pKa involves a logarithm!!!
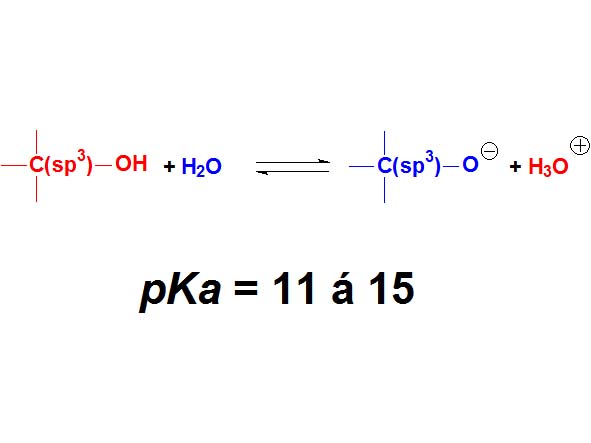 Alcohols
Alcohols
Believe it or not, the C=O groups induce a weak acidity in the neighbouring carbon.
Acetone displays a pKa = 20.
This is extremely weak an acidity but bases strong enough can get the proton in alpha position to the C=O group.
Ketones are not 'oxygen acids' but 'carbon acids'.
How many times is the acetone less acidic than a carboxylic acid as an average? Wrong again!!! It's not around 23 times but 10 to 23 times. Remember once again that the definition of pKa involves a logarithm!!!
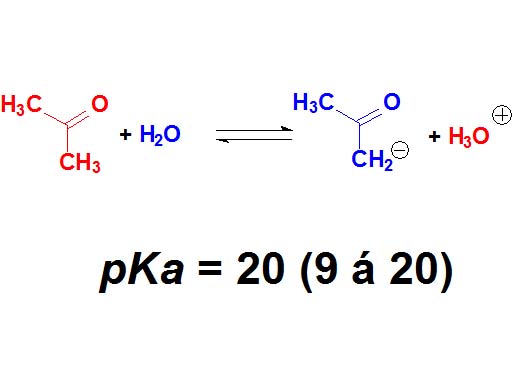 Ketones
Ketones
(Alpha C-H)
The stronger the acidity of a compound, the more shifted its equilibrium to the right hand side.
This fact suggests that the stronger the acidity of a compound, the more stable its conjugate base.
The more stable the conjugated base, the more shifted must the equilibrium be to the right hand side.
Carboxylate anion must be the most stable conjugate base of the four studied above.
This must be so because, experimentally, the corresponding acid displays the lowest pKa value of the four.
The exceptional stability of carboxylates can be explained by resonance forms.
The charge is highly and equaly delocalized between the two electronegative oxygens.
This suggests that delocalization is synonimous to stability.
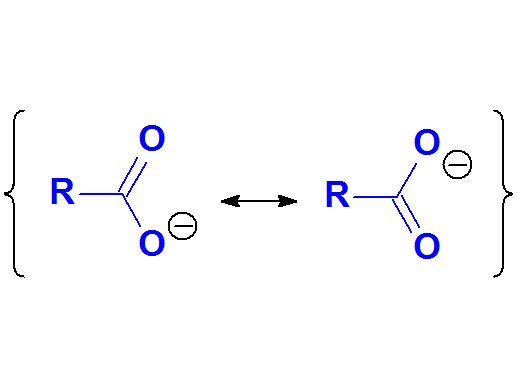 Carboxylate Anion
Carboxylate Anion
The Phenoxide anion must be a conjugated base less stable than a carboxylate but more than an alcoxide because it is so experimentally said by their pKa values.
Paradoxically, phenoxide can be described by more resonance forms than carboxylate but:
1) they are not equivalent;
2) the negative charge delocalizes to low-electronegative carbons.
These facts strongly suggest that phenoxide's 'quality' of its resonance forms are much lower than the carboxylate's two.
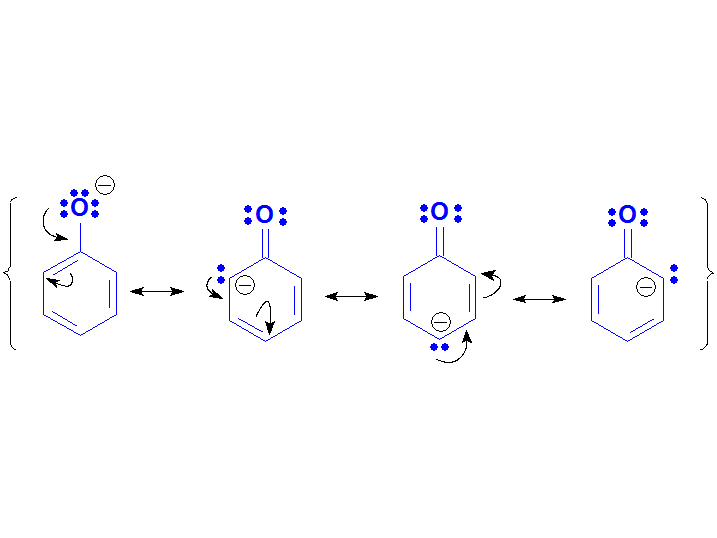 Phenoxide Anion
Phenoxide Anion
The alcoxide anion is an even lower stable conjugated base than the phenoxide because alcohols experimentally display higher pKa values than phenols.
Alcoxides CANNOT be described by resonance forms because THERE ARE NOT multiple bonds or lone electron pairs conjugated with the negatively charged O.
These facts strongly suggest that charge concentration over an atom is synonimous to unstability.
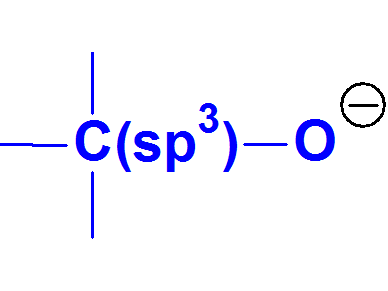 Alcoxide Anion
Alcoxide Anion
The enolate anion is the least stable conjugated base of the four due to the experimentally measured pKa value of, for example, acetone.
It can be described by two resonance forms but their 'quality' must be low.
This fact suggests that the negative charge concentration over a carbon is synonimous to high unstability, even though it can be delocalized towards an oxygen.
Despite the low acidity, many important reactions of carbonyl compounds take place through the formation of enolates
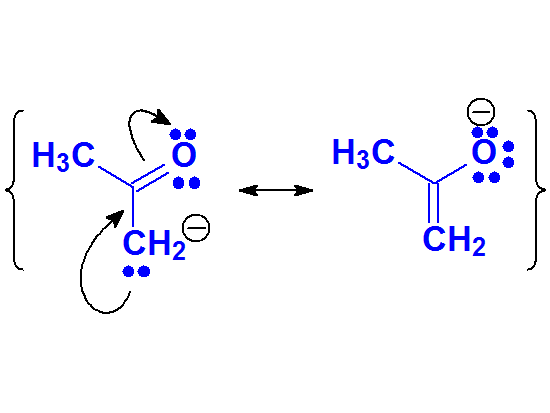 Enolate Anion
Enolate Anion
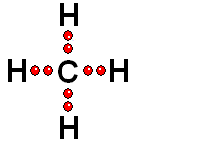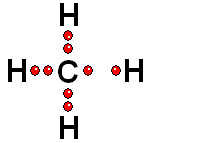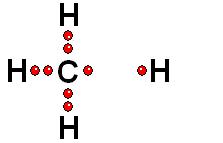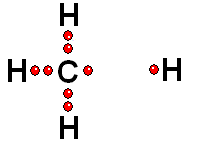
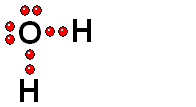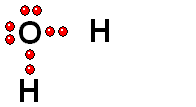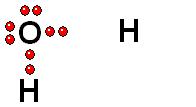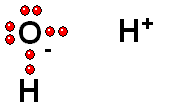
 Carboxylic Acids
Carboxylic Acids
 Phenols
Phenols
 Alcohols
Alcohols
 Ketones
Ketones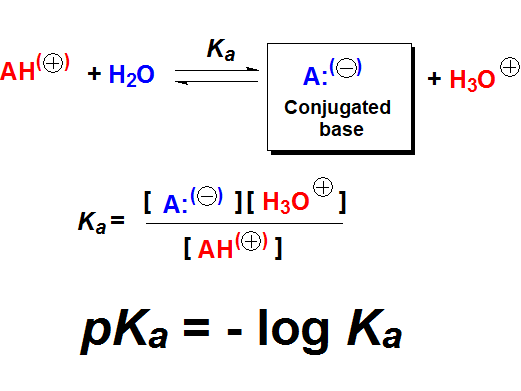
 Carboxylic Acids
Carboxylic Acids
 Phenols
Phenols
 Alcohols
Alcohols
 Ketones
Ketones Carboxylate Anion
Carboxylate Anion
 Phenoxide Anion
Phenoxide Anion
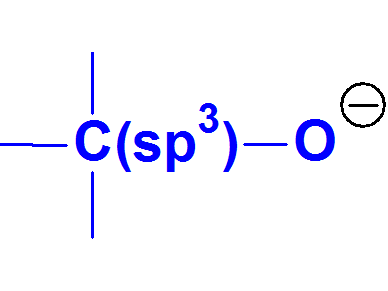 Alcoxide Anion
Alcoxide Anion
 Enolate Anion
Enolate Anion