Liebig in 1831 refined the method for experimentally determining centesimal compositions of carbon and hydrogen. Dumas discovers a method to determine nitrogen.

At the beginning of the 19th century, Berzelius developed an electrochemical theory on the formation of chemical compounds: attraction between opposite charges (dualistic theory). This theory is very successful among inorganic compounds but fails with organic ones. The difficulty definitely arises in understanding how such a vast number of organic compounds could be obtained from so few chemical elements (C, H, O, N). This difficulty and the fact that no organic compound had yet been prepared from inanimate matter, provokes the need to believe that organic compounds possess a vital force without which their existence is not possible. But, as we have seen, Wöhler accidentally synthesizes urea, a compound of animal origin, by reacting cyanogen with ammonium hydroxide in order to obtain ammonium cyanate, a compound of mineral origin. This discovery breaks the myth of the vital force since an organic substance could be prepared from mineral compounds. Gay-Lussac in 1815 studied prussic acid (hydrogen cyanide) and observed that the cyanide radical remained intact through a series of chemical transformations. Chemists of that time began to think of radicals as the organic-chemical equivalent of an element, hoping that the number of radicals would be limited, just as the number of elements was. However, the discovery of new radicals (methyl, ethyl, benzoyl, etc.) was explosive in the 1830s.

It is said that at the beginning of the 19th century it happened that, at a royal ball at the Tuileries Palace in Paris, the candles gave off an irritating vapor. Dumas was charged with finding out why and avoiding it. He concluded that these vapors were hydrogen chloride, which must have come from the fact that the candles had been bleached with chlorine, leaving a small amount of this element in them. Dumas determined with a series of experiments that some organic compounds react with chlorine, retaining part of it in their structure, releasing hydrogen chloride in the reaction. The properties of the chlorinated material were very similar to those of the starting product. As hydrogen was considered electropositive and chlorine electronegative, Berzelius' dualistic theory was unable to explain how hydrogen could be replaced by chlorine without also significantly altering the properties of the compound. Laurent presented his doctoral thesis in 1837 in which a three-dimensional model of molecular representation was described for the first time:

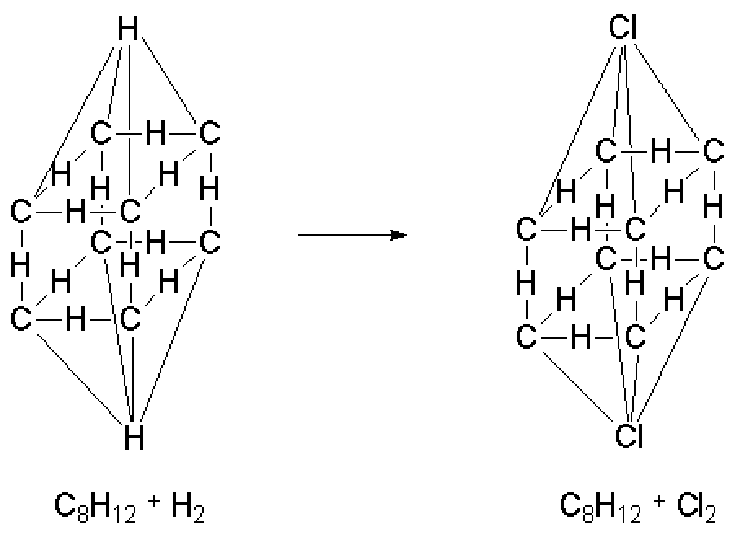
Dumas prepared trichloroacetic acid (1838) noting that the substitution of three hydrogens for as many chlorine atoms did not induce any appreciable change in the properties of the compound. However, in inorganic compounds, the replacement in an oxide of an electropositive atom with another electronegative one almost invariably leads to a drastic change in its acid-base properties. The idea is taking shape that the chemical properties of an organic compound do not depend only on the nature of the elements that form it but also on their spatial arrangement, which clashes head-on with the dualist theory.