In 1850 Williamson reacted alcohols with potassium and then with alkyl iodides, preparing ethers through the important substitution reaction that bears his name. He extended this global replacement of hydrogen by an alkyl residue to acids and obtained esters. He predicted the formation of anhydrides by replacing the hydrogen of an acid with an acyl moiety.

In 1852 Gerhardt obtained the first anhydride of a monocarboxylic acid following the procedure predicted by Williamson: the reaction of acetyl chloride with potassium acetate. All these investigations led alcohols, ethers, acids, esters and anhydrides to be considered within an inorganic type, that of water, from which they are derived by replacing one or two of its hydrogens with organic radicals. Gerhardt added two other types of molecules to the list: those derived from hydrogen (hydrocarbons) and hydrogen chloride (alkyl halides). The grouping of some of the organic molecules into this increasing number of types was fundamental to the elucidation of the spatial arrangement of atoms in organic compounds.

In 1852 Frankland published an article establishing that nitrogen, phosphorus, arsenic and antimony always bind to 3 or 5 other atoms or species, establishing the basis of the current idea of atomic valence. Chemists at that time were not yet aware that the number of bonds that an atom could form was limited. In 1855 Odling proposed the methane type based on the idea that chloroform is a derivative of it.

Kekulé, unaware of Odling's work, added the methane type to the previous types in 1857 and published a work where he clearly established the four valences of carbon. A year later (1858) he published the work laying the foundations for modern structural organic chemistry, although without using any graphic molecular formula. The following year (1859) he wrote a textbook where his famous sausage-type formulas appeared.
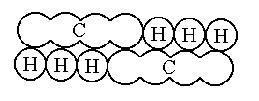 CH3CH3 ethane
CH3CH3 ethane
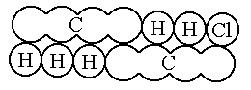 CH3CH2Cl ethyl chloride
CH3CH2Cl ethyl chloride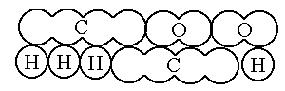 CH3COOH acetic acid
CH3COOH acetic acid

A month later than Kekulé's work (1858), Couper gets Dumas to publish a work in which, independently, the same principles expressed by Kekulé are explained, although in a slightly different way using primitive formulas. He concludes his work with the first organic formula where the formation of a cycle is proposed.
