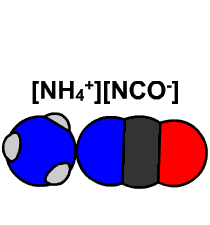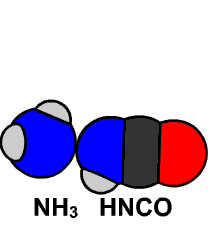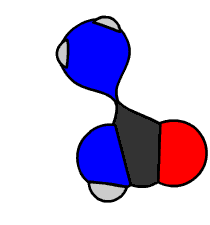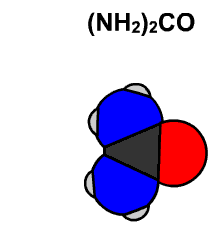
Friedrich Wöhler managed to synthesize an organic compound, urea, from an inorganic compound, thus putting an end to the vitalist theory of Jöns Jacob Berzelius. Wöhler discovered that urea and ammonium cyanate have the same atomic composition, but very different chemical properties. It can be said that this fact marks the discovery of isomerism, since urea and ammonium cyanate have different formulas, CO(NH2)2 and NH4CNO, respectively, and the same elemental composition.

Around 1820 Elemental Analysis became a standard technique in Chemistry. The most relevant researcher in this technique was William Prout. In 1817 he carried out the analysis of urea, obtaining 46.65% nitrogen, 26.65% oxygen, 19.975% carbon and 6.67% hydrogen. Modern values are 46.67% N, 26.67% O, 20.0% C, and 6.67% H.
